Irene H. Heijink
Resume
Professor, Tenure-track Associate Professor, University of Groningen, The Netherlands, 2015 –
Lead of EXPIRE Group, Departments of Pulmonology and Pathology & Medical Biology, University of Groningen, The Netherlands, 2013 –
Tenure Track, University of Groningen, University Medical Center Groningen, The Netherlands, 2011 –
Post-doctoral position, University of Groningen, The Netherlands, 2009
PhD, Departments of Allergology, Hematology and Pulmonology, University of Groningen, The Netherlands, 2004
Thesis title: Dysregulation of T cell activity in asthma; role of the β2-adrenergic/cAMP system
BSc, University of Groningen, Medical Biology, Faculty of Mathematics and Natural Sciences, 1998
Research
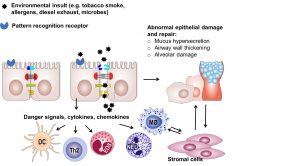
The main focus of my research is the damage and repair of the immunological mucosal barrier in lung disease. After my initial interest in the regulation of immune responses and interaction between T cells and the structural epithelial cells of the lung, my focus shifted towards the regulatory role of the epithelium in asthma and COPD. I studied a novel role of airway epithelial plasticity in these pathologies, showing that loss of epithelial barrier function is critical in the decision towards immunity and that epithelial-to-mesenchymal may drive tissue remodeling in these diseases. New concepts have emerged from these studies, e.g. on the role of mitochondrial dysfunction in abnormal airway epithelial responses in COPD. In addition, a crucial role has been proposed for epithelial damage and epithelium-derived danger signals in the pathogenesis of COPD.
My research is mainly performed using molecular approaches and cellular models in a highly translational setting. Close collaboration with clinicians and pathologists has enabled me and my team to create a unique biobank of lung epithelium and mesenchymal stromal cells for the use of patient-specific models, including the recently set-up human airway and alveolar organoid models. These advanced 3D models support our understanding of the mechanisms underlying abnormal lung epithelial repair and regeneration, in order to gain more insight in novel therapies to repair the damaged epithelium. This includes studies on mesenchymal stem/stromal cells. I have an extensive network, including ongoing collaborations with amongst others the University of Ghent, the University of British Columbia (Vancouver, Canada) and the University of Sydney (Australia).
Samarelli, AV, Tonelli, R, Heijink, I, Martin Medina, A, Marchioni, A, Bruzzi, G et al.. Dissecting the Role of Mesenchymal Stem Cells in Idiopathic Pulmonary Fibrosis: Cause or Solution. Front Pharmacol. 2021;12 :692551. doi: 10.3389/fphar.2021.692551. PubMed PMID:34290610 PubMed Central PMC8287856.
Pouwels, SD, Wiersma, VR, Fokkema, IE, Berg, M, Ten Hacken, NHT, van den Berge, M et al.. Acute cigarette smoke-induced eQTL affects formyl peptide receptor expression and lung function. Respirology. 2021;26 (3):233-240. doi: 10.1111/resp.13960. PubMed PMID:33078507 PubMed Central PMC7983955.
Faura Tellez, G, Willemse, BW, Brouwer, U, Nijboer-Brinksma, S, Vandepoele, K, Noordhoek, JA et al.. Protocadherin-1 Localization and Cell-Adhesion Function in Airway Epithelial Cells in Asthma. PLoS One. 2016;11 (10):e0163967. doi: 10.1371/journal.pone.0163967. PubMed PMID:27701444 PubMed Central PMC5049773.
Ojo, O, Lagan, AL, Rajendran, V, Spanjer, A, Chen, L, Sohal, SS et al.. Pathological changes in the COPD lung mesenchyme–novel lessons learned from in vitro and in vivo studies. Pulm Pharmacol Ther. 2014;29 (2):121-8. doi: 10.1016/j.pupt.2014.04.004. PubMed PMID:24747433 .
Heijink, I, van Oosterhout, A, Kliphuis, N, Jonker, M, Hoffmann, R, Telenga, E et al.. Oxidant-induced corticosteroid unresponsiveness in human bronchial epithelial cells. Thorax. 2014;69 (1):5-13. doi: 10.1136/thoraxjnl-2013-203520. PubMed PMID:23980116 .
Lo Tam Loi, AT, Hoonhorst, SJ, Franciosi, L, Bischoff, R, Hoffmann, RF, Heijink, I et al.. Acute and chronic inflammatory responses induced by smoking in individuals susceptible and non-susceptible to development of COPD: from specific disease phenotyping towards novel therapy. Protocol of a cross-sectional study. BMJ Open. 2013;3 (2):. doi: 10.1136/bmjopen-2012-002178. PubMed PMID:23377993 PubMed Central PMC3586075.
Search PubMed